Compiled by Walter Sorochan
Posted July 03, 2011
Growing replacement
organs has been one of the "maybe someday" fantasies of doctors for a
generation. Gone would be long waiting lists for transplants -- along with
complications arising from tissue rejection. In 2006, medical researchers
led by Anthony Atala and Alan Retik announced that they had largely regrown
bladders using their patients' own cells, then implanted them. Science
fiction had become fact.
 Surgeons typically repair disease-damaged bladders using intestinal tissue
from a patient's own body, but the procedure often leads to complications,
and even cancer. "What really bothered me was seeing the practice done in
children, who would be plagued by problems throughout their lives," says
Atala, director of the Institute of Regenerative Medicine at Wake Forest
University Baptist Medical Center.
Surgeons typically repair disease-damaged bladders using intestinal tissue
from a patient's own body, but the procedure often leads to complications,
and even cancer. "What really bothered me was seeing the practice done in
children, who would be plagued by problems throughout their lives," says
Atala, director of the Institute of Regenerative Medicine at Wake Forest
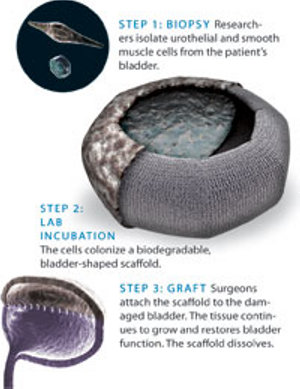
University Baptist Medical Center. Doctors led by Retik, chief of urology at the Children's Hospital in Boston, took bladder biopsies from patients. The urothelial cells of the inner layer were separated from cells of the outer layer of muscle, and cultured. Then, researchers plaited the cells onto a spongelike biodegradable scaffold, made of a synthetic polymer and collagen, in the shape of a bladder. After a seven-week incubation period, surgeons grafted the new bladder/scaffold segments onto the patients' damaged bladders. All seven patients improved -- and are continuing to thrive.
Atala's research team at Wake Forest is now growing 20 different tissues, including heart patches, pancreatic insulin-producing tissue and kidney tissue. The researchers still have much to learn, but hopes remain high. They recently received funding from the Department of Defense for work that could one day lead to regenerated limbs for injured soldiers. Ward: Grow organs
Interview below with Anthony Atala, director of the Wake Forest Institute for Regenerative Medicine Haake: How to Grow New Organs
![]() Question: How do you build a human liver that could one day function normally inside a patient?
Question: How do you build a human liver that could one day function normally inside a patient?
![]() Answer: We've been working on the concept of building solid organs for quite
some time now, and we have many different strategies. One strategy
is to take a donor organ that's not being used and would have been
discarded and, using very mild detergents, wash the cells away.
Basically, two weeks later, you're left with something that looks a
liver, you can hold it like a liver, it feels like a liver, but it
has no cells. It's just a skeleton of the liver, the matrix, which
we call the scaffold. Using that technology, we're able to reperfuse
that scaffold with, ideally, the patient's own cells.
Answer: We've been working on the concept of building solid organs for quite
some time now, and we have many different strategies. One strategy
is to take a donor organ that's not being used and would have been
discarded and, using very mild detergents, wash the cells away.
Basically, two weeks later, you're left with something that looks a
liver, you can hold it like a liver, it feels like a liver, but it
has no cells. It's just a skeleton of the liver, the matrix, which
we call the scaffold. Using that technology, we're able to reperfuse
that scaffold with, ideally, the patient's own cells.
![]() If using the patient's own cells is ideal, what happens when that's not possible?
If using the patient's own cells is ideal, what happens when that's not possible?
![]() Luckily, for the most part, we're able to use cells from the same
organ, even if that organ is deceased. We use the progenitor cell
population: the cells that have the potential to form the organ but
have not been affected by the death of the organ. We like to use
these cells because they're organ-specific cells and already know
what to do. If you're going to make a windpipe, you want cells that
already know that they're a windpipe.
Luckily, for the most part, we're able to use cells from the same
organ, even if that organ is deceased. We use the progenitor cell
population: the cells that have the potential to form the organ but
have not been affected by the death of the organ. We like to use
these cells because they're organ-specific cells and already know
what to do. If you're going to make a windpipe, you want cells that
already know that they're a windpipe.
![]() What if the organ needing to be implanted in the patient just isn't
available?
What if the organ needing to be implanted in the patient just isn't
available?
![]() We very rarely have a case where you are missing cells from the
organ in question, but if we don't have organ-specific cells, the
next best choice would be stem cells from other parts of the
patient's body. For example, if you're trying to make a liver, you
may be able to get stem cells from the bone marrow to re-create the
tissue of the liver. In this case, since you get the cells from the
same body, there's no rejection.
We very rarely have a case where you are missing cells from the
organ in question, but if we don't have organ-specific cells, the
next best choice would be stem cells from other parts of the
patient's body. For example, if you're trying to make a liver, you
may be able to get stem cells from the bone marrow to re-create the
tissue of the liver. In this case, since you get the cells from the
same body, there's no rejection.
![]() The liver isn't the first organ you've built in the lab—you've
successfully implanted a lab-grown bladder. So what makes this
development particularly noteworthy?
The liver isn't the first organ you've built in the lab—you've
successfully implanted a lab-grown bladder. So what makes this
development particularly noteworthy?
![]() Building a solid organ like the liver in the lab is different and
harder than with an organ like the bladder because solid organs are
very vascular. When we process the liver, we actually have to
preserve the blood vessel tree and perfuse it with blood vessel
cells. Then we infiltrate the parenchyma of the scaffold with the
liver cells. It's much more complicated. It's the difference between
baking a cake and making a peanut butter and jelly sandwich.
Building a solid organ like the liver in the lab is different and
harder than with an organ like the bladder because solid organs are
very vascular. When we process the liver, we actually have to
preserve the blood vessel tree and perfuse it with blood vessel
cells. Then we infiltrate the parenchyma of the scaffold with the
liver cells. It's much more complicated. It's the difference between
baking a cake and making a peanut butter and jelly sandwich.
![]() Tell me a little about how these lab-grown organs are made. Is it a
long process?
Tell me a little about how these lab-grown organs are made. Is it a
long process?
![]() We start out by getting a cell tissue box from the patient, usually
less than half the size of a postage stamp. Then we extract the
cells from that piece of tissue to expand the cells outside the body
in large quantities. Then we start composing the tissue very much
like a layered cake, one layer at a time. Doing this, we're able to
create the organ, and we put it into this oven-like device that has
the same conditions as the human body—37 degrees Celsius and 95
percent oxygen. We let the organ "cook" here, and usually two weeks
after, that the organ is ready for implantation. So it takes about
four weeks to grow the cells and about two weeks to create the
organ. We usually give ourselves about eight weeks for the entire
process.
We start out by getting a cell tissue box from the patient, usually
less than half the size of a postage stamp. Then we extract the
cells from that piece of tissue to expand the cells outside the body
in large quantities. Then we start composing the tissue very much
like a layered cake, one layer at a time. Doing this, we're able to
create the organ, and we put it into this oven-like device that has
the same conditions as the human body—37 degrees Celsius and 95
percent oxygen. We let the organ "cook" here, and usually two weeks
after, that the organ is ready for implantation. So it takes about
four weeks to grow the cells and about two weeks to create the
organ. We usually give ourselves about eight weeks for the entire
process.
![]() How does the ability to grow organs (and tissues) change the current
state of medicine?
How does the ability to grow organs (and tissues) change the current
state of medicine?
![]() There are more than 110,000 people currently on U.S. transplant
waiting lists. And with current technologies, patients, for the most
part, have to wait for someone else to die before they can get an
organ and live, which is kind of ironic if you think about it. Every
day, patients are waiting for organs, and they're getting worse and
worse over time.
There are more than 110,000 people currently on U.S. transplant
waiting lists. And with current technologies, patients, for the most
part, have to wait for someone else to die before they can get an
organ and live, which is kind of ironic if you think about it. Every
day, patients are waiting for organs, and they're getting worse and
worse over time.
And, when a patient does get an organ from another person, it comes from a different body. It has different properties, and a person's natural tendency is to reject that organ. So the two major challenges today are tissue and organ rejection and shortage. That is where this field called regenerative medicine comes in, [bringing] together many different specialty areas to achieve a way to create ready-made organs and tissues for patients that are made from the patient's own cells.
![]() You implanted the first lab-grown bladder into a patient already.
How's she doing?
You implanted the first lab-grown bladder into a patient already.
How's she doing?
![]() She's been doing very well. It's been four or five years since we
published the paper, but she's had the bladder for over 10 years.
She's been doing very well. It's been four or five years since we
published the paper, but she's had the bladder for over 10 years.
![]() So what are the next steps for your work on the liver?
So what are the next steps for your work on the liver?
![]() Right now we're making miniature livers. We're in the process of
constructing larger segments that are at least four times as large
as the current ones. Our ultimate goal is to make the livers larger
and larger until we can get them to a size that can be implanted
into a patient.
Right now we're making miniature livers. We're in the process of
constructing larger segments that are at least four times as large
as the current ones. Our ultimate goal is to make the livers larger
and larger until we can get them to a size that can be implanted
into a patient.
![]() What are the main obstacles to achieving this goal?
What are the main obstacles to achieving this goal?
![]() The main obstacle is the vascular aspect of the organ—translating it
to work in larger structures while preserving the same paradigm you
see in the small livers
The main obstacle is the vascular aspect of the organ—translating it
to work in larger structures while preserving the same paradigm you
see in the small livers
![]() Is it possible that we can build a heart?
Is it possible that we can build a heart?
![]() Yeah, we're actually using the same strategy to build the heart as
we are with the liver.
Yeah, we're actually using the same strategy to build the heart as
we are with the liver.
![]() Can we expect to see miniature hearts soon then?
Can we expect to see miniature hearts soon then?
![]() Absolutely, yes. We're building the heart currently using the human
cells.
Absolutely, yes. We're building the heart currently using the human
cells.
![]() What about brains?
What about brains?
![]() Absolutely. I think this same strategy could be applied to build
most solid organ systems in the body.
Absolutely. I think this same strategy could be applied to build
most solid organ systems in the body.
![]() Wow. So what kind of things would be transferred over? For example,
when someone suffering from brain trauma underwent a brain implantation,
would memories and skills learned over time remain intact?
Wow. So what kind of things would be transferred over? For example,
when someone suffering from brain trauma underwent a brain implantation,
would memories and skills learned over time remain intact?
![]() The goal for us is to reproduce as many properties as possible. We
test these organs at all levels—the molecular biology, cell biology,
physiology and pharmacology levels—before we implant them to make
sure that they function the same way a human organ does.
The goal for us is to reproduce as many properties as possible. We
test these organs at all levels—the molecular biology, cell biology,
physiology and pharmacology levels—before we implant them to make
sure that they function the same way a human organ does.
![]() So a lab-grown brain might function similarly to the brain of a
newborn baby?
So a lab-grown brain might function similarly to the brain of a
newborn baby?
![]() That's right.
That's right.
![]() Do you ever see a day when we'll have mass-produced lab organs?
Do you ever see a day when we'll have mass-produced lab organs?
![]() The future is certainly pointing in a direction. [But], since they
come from the patient's cells, we'll still be making them
individually.
The future is certainly pointing in a direction. [But], since they
come from the patient's cells, we'll still be making them
individually.
References:
Altman Lawrence, "On a Scaffold in the Lab, Doctors Build a Bladder," New York Times, April 4, 2006. Altman: new bladder
Haake Allie, "How Doctors Will Build Your New Liver (or Heart or Brain) in the Lab," Atala Anthony, Wake Forest Institute for Regenerative Medicine, Popular Mechanics, April 25, 2011. Haake: How to Grow New Organs
Ward Logan, "Beyond Transplants: Growing Organs in the Lab," Popular Mechanics, December 18, 2009 Innovators Anthony Atala and Alan Ward: Grow organs