Adapted by Walter Sorochan from original info by John Cannell - Vitamin D Council
Posted May 02, 2010; updated October 17, 2021.
Your hunger and passion to eat illustrates the body's need for food or fuel everyday. But the inside body cannot use the raw food we ingest. The food needs to be broken down so that vitamins, minerals, proteins, fats and carbohydrates can be made available to the body through a further in a series of biochemical reactions.
Biochemical reactions in the human body need to occur instantly for humans and other living organisms to function and survive. For such inner functions to occur rapidly, mother nature has evolved a series of chemical transactions aided by cofactors and coenzymes. A simplistic example is the use of enzymes in the digestive system to break down our food so the micronutrients can be absorbed from the digestive system into the blood stream. On a smaller scale, such tear downs and rebuilding of substances occur in nano-time by cofactors and coenzymes. Otherwise, the great majority of reactions required by humans would occur far too slowly to be of any use.
This article attempts to simplify, summarize and illustrate a few of the co-factors and co-enzymes relevant to good health. Vitamins and minerals need each other and they need to be in proper ratios! Sad truth is that there is very little research going on to unravel these reactions and ratio mysteries!
Three major issues about food need to be resolved:
These three keys to better health are essential for the public being able to use supplements properly. Medical science has ignored giving a high priority to research these three key issues.
Before proceeding further, we need to define a few key terms:
- Enzymes: do the work of a cell . They act to increase the rate at which body reactions occur [ putting together or tearing them apart ].
- Coenzymes: mostly derived from vitamins, are organic molecules that are required by certain enzymes to carry out catalysis [ cause reactions to occur faster and require less activation energy ].
- Cofactors: are usually metallic ions, often classified as inorganic substances that are required for, or increase the rate of, catalysis. Cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical reactions. These cofactors appear to be super-switches for energy production and balance, growth, development, reproduction, survival, and life extension.
So .... definitions do not explain how co-enzymes and co-factors work! But the video below can help you do so. Paul Andersen explains how enzymes are used to break down substances like food in the body:
Source:
Andersen: enzymes
Thousands of enzymatic reactions require metal ion co-factors in functions. Metals iron, zinc, copper, nickel, vanadium, tungsten, manganese, molybedium, chromium, and magnesium provide functionality to enzymes and are referred to as metal ion cofactors. These metalic ions are often bound inside amino acids. Christensen: Amino Acids to Dopamine & Serotonin 2015
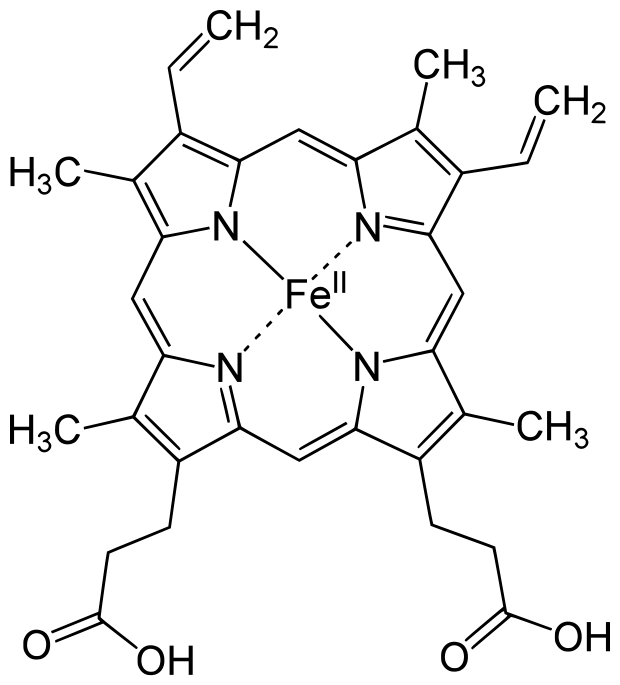 Example:
Iron is key player in transport of oxygen; but is helped by copper and cobalt.
The illustration on the right displays the protein hemoglobin with the metallic
iron in the middle. Hemoglobin [Hb or Hgb] is the iron-containing oxygen-transport metal-protein in the red
blood cells of all vertebrates. Hemoglobin in the blood carries oxygen from the
respiratory lungs to the rest of the body.
Example:
Iron is key player in transport of oxygen; but is helped by copper and cobalt.
The illustration on the right displays the protein hemoglobin with the metallic
iron in the middle. Hemoglobin [Hb or Hgb] is the iron-containing oxygen-transport metal-protein in the red
blood cells of all vertebrates. Hemoglobin in the blood carries oxygen from the
respiratory lungs to the rest of the body.
Mineral deficiency in humans can disrupt normal body processes.. Although chromium deficiency causes impaired glucose tolerance, no human enzyme that uses this mineral as a cofactor has been identified. Another example is deficiency of iodine. Iodine can cause goiter; although this element is used as part of the structure of thyroid hormones rather than as an enzyme cofactor. Calcium is another special case, in that deficiency can cause osteoporosis and a host of other misunderstood health problems. Calcium is, therefore, a cell signaling molecule, and not usually considered a cofactor of the enzymes it regulates.
Co-factors are " helper molecules " that help other nutrients become active and functional. We know very little about co-factors and how these minerals affect general health. Missing in the co-factor picture is the exact ratio of co-factors to each other to help the body function at its best. The exact ratio is usually an educated guess!
Although co-factors are an essential part of human metabolism, there are other important factors that affect how co-factors work. The most important of these factors is the biochemistry of each individual or biochemical individuality. "One man's meat is another man's poison!" Variable biochemistry is least accounted for in research studies. Another factor affecting the outcome of nutrients are that co-factors work in unison as a total package. That is, all must be present in specific and appropriate ratios in order to be effective helpers of optimal metabolism and body functions. Another exception of co-factor function are minerals usually working in pairs. e.g. Ca:Mg and Iron:copper:cobalt.
Cofactors are usually minerals but how these work in humans is complex and difficult to understand.
Examples of some enzymes that require metal ions as cofactors is shown in the:
| cofactor | enzyme or protein |
| Zn++ | carbonic anhydrase |
| Zn++ | alcohol dehydrogenase |
| Fe+++ or Fe++ | cytochromes, hemoglobin |
| Fe+++ or Fe++ | ferredoxin |
| Cu++ or Cu+ | cytochrome oxidase |
| K+ and Mg++ | pyruvate phosphokinase |
Co-enzyme is an organic molecule that is required by certain enzymes to carry out the break down [catalysis] of substances like nutrients [ a substrate ]. Co-enzymes bind to the active site of the enzyme, participate in the catalysis but are not part of the substrate. There is usually an electron change that facilitates a reaction change.
Obviously, one cannot discuss co-factors and co-enzymes without discussing catalysis and the chemical reactions of reduction and oxidation. So a momentary diversion to fill in this essential information.
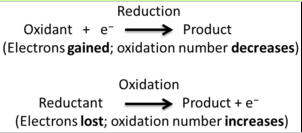 Catalysis involves redox reactions
that are about electron exchange; a matched set .... that is, there is a chemical reduction and and oxidation taking place.
Oxidation takes place with loss of electrons; while reduction gains electrons.
There cannot be an oxidation reaction without a reduction reaction happening simultaneously.
The oxidation alone and the reduction alone are each called a half-reaction, because two half-reactions always occur together to form a whole reaction.
Co-enzymes make oxidation and reduction happen, resulting in energy.
Catalysis involves redox reactions
that are about electron exchange; a matched set .... that is, there is a chemical reduction and and oxidation taking place.
Oxidation takes place with loss of electrons; while reduction gains electrons.
There cannot be an oxidation reaction without a reduction reaction happening simultaneously.
The oxidation alone and the reduction alone are each called a half-reaction, because two half-reactions always occur together to form a whole reaction.
Co-enzymes make oxidation and reduction happen, resulting in energy.
Below is an example of redux reaction using an apple by Jack Hancock: Length = 4:11 mns.
Source: Video apple
In human metabolism, a co-factor may be illustrated by a natural co-enzyme, nicotinamide adenine dinucleotide or NAD+, found in living cells. In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another.
"The co-enzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery. In organisms, NAD can be synthesized from simple building-blocks from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the co-enzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related co-enzyme is similar to that of NAD, but it has different roles in metabolism." Wiki: NAD
Coenzyme - cofactor examples: Vitamin D works best when magnesium, zinc, vitamin K2, vitamin A and boron are available, in proper amounts, at the same time in the body. The mineral co-factors are bound together with amino acids to form metallic-enzymes. Vitamins also combine with amino acids to form co-enzymes. Both metallic cofactors and vitamin enzymes assist in metabolic transactions. That is, they help vitamin D function in the body by increasing and speeding up the chemical reactions in the body. There is an interdependent synergistic action among nutrients. No nutrient acts in a vacuum or by itself. [ Yet, this is the assumption of many persons, doctors and nutritionists today ]
Illustrations Co-factors in action: 'Cofactor' simply means another nutrient that's required for the efficient production and use of vitamin D. These include: Magnesium, Zinc, Vitamin K2, Vitamin A and Boron. Magnesium (Mg) can act as a cofactor in combination with vitamin D. It is the fourth most abundant mineral in the body and is involved in more than 300 biochemical reactions. All the enzymes that metabolize Vitamin D require Mg. It is also required in each of the steps concerned with replication, transcription and translation of genetic information, and thus it is also needed for the genetic action of Vitamin D. Cannell: Vitamin D council: Article no longer active. Carpenter: Info Mg 1988 Zofkova Mg Ca hormones
Example 1 Vit D: Two interesting cases of Mg dependent Vitamin D-resistant rickets appeared in the Lancet in 1974. Two children, one age two and the other age five, presented with classic rickets. 600,000 IU of Vitamin D daily for ten days did not result in any improvement in six weeks—in either x-rays or alkaline phosphate and the doctors diagnosed Vitamin D-resistant rickets. Almost by accident, serum Mg levels were then obtained, which were low in both children. After the treatment with Mg, the rickets rapidly resolved. Cannell: Vitamin D council: Article no longer active. Reddy: MG and rickets 1974
"What does all this mean? How can one treat rickets with Mg? Remember, these children took a total of 6 million units, that's a total of 6,000,000 IU of vitamin D over ten days [ it was given as injections so we know the children actually took it ]. Thus, they had plenty of vitamin D but, in their cases, the vitamin D needed Mg to work." Reddy: MG and rickets 1974
Example 2 Calcium: In 1976, Dr. Ramon Medalle and colleagues at the Washington University School of Medicine described five patients with Mg deficiency and low blood calcium [ whose calcium blood levels would not return to normal after vitamin D treatment [ a condition known as Vitamin D resistance ]]. However, serum calcium promptly returned to normal in all five patients after treatment with Mg, raising the possibility that such Vitamin D resistance may be caused from simple, but severe, Mg deficiency. Cannell: Vitamin D council: Article no longer active. Mendalle: NIH Mg more info 1976
Another example of calcium using a protein to do its work is Calmodulin (CaM) [an abbreviation for calcium-modulated protein]. Calmodulin is a calcium-binding messenger protein expressed in all plant and animal [eukaryotic] cells. Calmodulin is a multifunctional intermediate messenger protein that transduces calcium signals by binding calcium ions and then modifying its interactions with various target proteins. Wiki: Calmodulin
The missing information about calcium is the amount of vitamin D and magnesium [ ratios] needed to help calcium do its work. Current thinking about all of this is that ratios may be dependent on bio-diversity of individuals. If this is true, then one would need to adjust by guessing the nutrient supplement-mineral ratios to fit their biological background. Tinkering with nutritional status using supplements is potentially problematic. Despite what some people might tell you, our understanding of nutrition and human health is currently rather crude and most incomplete.
Example 3 Iron: Co-factors for good iron absorption and utilization are copper and cobalt. Anemia, which is a deficiency in iron, is one of the more commonly found deficiencies. It may be caused either by not getting enough iron [ due to not enough copper or cobalt ] in one's diet or by an inability of the body to properly absorb. Vitamin C facilitates iron absorption. Frantom: iron & chemistry 2006
Example 4 Chromium: is a trace mineral that helps insulin work more efficiently. Chromium cofactor ininsulin The human insulin protein is composed of 51 amino acids.
Example 5 Vit D3: [ vit D 25(OH) ] Vit D3 increases the level of calcium (Ca2+) in the blood by:
(1) increasing the uptake of calcium from the gut into the blood,
(2) decreasing the transfer of calcium from blood to the urine by the kidney, and
(3) increasing the release of calcium into the blood from bone. Wiki Vit D3 and Ca
Example 6 Selenium: deficiency can exacerbate the effects of iodine deficiency. Iodine is essential for the synthesis of thyroid hormone, but selenium-dependent enzymes (iodothyronine deiodinases) are also required for the conversion of thyroxine (T4) to the biologically active thyroid hormone, triiodothyronine (T3). Additionally, deficiencies of vitamin A or iron may exacerbate the effects of iodine deficiency : Linus Pauling Institute Selenium is synergistic with vitamin E. Together they are more powerful than the sum of both combined.
Example 7 Iodine: Scientists do not have a clear picture of just how each of the co-factors [ selenium, iron, vitamin C ] work in helping iodine do its job. But scientists have found that thyroid metabolism is controlled locally in the tissue by each organ. That is, the brain has one mechanism for controlling the amount of thyroid available to the brain but it is different from other tissues such as the liver. There are many mechanisms by which each tissue controls the amount of thyroid hormone which gets into the tissues. But to discuss one: there is an enzyme in the tissue which deiodinates [takes one iodine off the thyroxine T4] and makes T3 or triiodothyronine. These enzymes are called deiodinases. Every tissue has different types of deiodinases. Shomon: Derry interview
Example 8 Organic Sulfur: Upon exposure to the sun, the skin synthesizes vitamin D 3 sulfate, a form of vitamin D that, unlike unsulfated vitamin D 3, is water soluble. Cholesterol sulfate is also synthesized in the skin, where it forms a crucial part of the barrier that keeps out harmful bacteria and other microorganisms such as fungi. Red blood cells are strong producers of cholesterol sulfate, as well as major carriers of oxygen. Sulfur is also a component of insulin, the hormone that regulates carbohydrate metabolism. For more details go to: Sorochan: sulfur
Example 9 Vitamin K2: Vitamin K2 is an essential helper to calcium, vitamins A, D and C in numerous body functions. Vitamin K acts as a coenzyme in helping other nutrients function in the body. Mercola: Interview with Dr. Vermeer "Vitamin K is the substance that makes the vitamin A- and vitamin D-dependent proteins come to life. While vitamins A and D act as signaling molecules, telling cells to make certain proteins, vitamin K activates these proteins by conferring upon them the physical ability to bind calcium. Masterjohn: History of vit K 2008
Example 10 Serotonin: "Serotonin is produced in your brain and gastrointestinal tract from the enzymatic conversion of the amino acid L-tryptophan. In this two-step process, L-tryptophan is converted to 5-hydroxy-L-tryptophan, or 5-HTP, by an enzyme called tryptophan hydroxylase, or TPH. TPH requires tetrahydrobiopterin, a nitrogen-containing cofactor, to perform this initial step. In the second step, another enzyme called amino acid decarboxylase, or AADC, converts 5-HTP to serotonin. According to a 1995 “Journal of Neural Transmission” study, the activity of AADC is enhanced by pyridoxal-5-phosphate, an activated form of vitamin B-6". Christensen: Amino Acids to Dopamine & Serotonin 2015
Seratonin [ enzyme tyrosine hydroxylase ] and dopamine [ enzyme tryptophan hydroxylase ] both contain iron. Magnesium and zinc help to potentiate the activities of serotonin and dopamine but are not intimately involved in neurotransmitter synthesis. Christensen: Amino Acids to Dopamine & Serotonin 2015
Example 11 Then there are Foods labeled as Goitrogens: Some foods contain substances that interfere with iodine utilization or thyroid hormone production; these substances are called goitrogens. The occurrence of goiter in the Democratic Republic of Congo has been related to the consumption of casava, which contains a compound that is metabolized to thiocyanate and blocks thyroidal uptake of iodine. Some species of millet and cruciferous vegetables [for example, cabbage, broccoli, cauliflower, and Brussels sprouts] also contain goitrogens. Furthermore, the soybean isoflavones, genistein and daidzein, have been found to inhibit thyroid hormone synthesis. Most of these goitrogens are not of clinical importance unless they are consumed in large amounts or there is coexisting iodine deficiency. Recent findings also indicate that tobacco smoking may be associated with an increased risk of goiter in iodine-deficient areas. The chemicals in goitrogens can be neutralized by boiling or steaming the food.
Example 12 Minerals & amino acids: Minerals need help to be absorbed from the small intestine into the blood and function in the body. They get this help from many proteins [ amino acids ] acting as enzymes that catalyze the chemical reactions in metabolism. Frantom: iron & chemistry 2006
Although mineral supplements do combine a mineral with amino acid, we get almost no information about this on the supplement labels. Consumers do not know how much is really absorbed into the blood stream and from the blood stream to the somatic cells.
Example 13 Electrolytes: Potassium and sodium are essential for transmission of nerve impulses throughout the body. Potassium is involved in muscle contraction, including the heart, and thereby helps to maintain blood pressure in the normal range. Potassium works with sodium to maintain the body's water balance; but the appropriate mineral ratio is probably a guess.
Best Sources of co-factors: The best way to ensure that we get co-factors used to be from a balanced diet. But with mineral depleted soil, our food is fast becoming depleted in adequate and essential minerals. We are becoming more and more dependent on mineral supplements.
There are now supplements on the market that 'claim' to contain all the co-factors vitamin D needs to work properly [including magnesium], zinc is contained in the base of the vitamin D Receptor, Vitamin K2 helps direct Vitamin D to calcify the proper organs and prevents calcification of improper organs, boron is involved in the rapid, non-genomic action of Vitamin D on the cell wall), a small amount of genestein [ about one-half the amount the average Japanese consumes every day ] helps activate vitamin D to stay around longer at the receptor site. Cannell: Vitamin D council: Article no longer active.
These few examples of co-factors in action are by no means a complete picture. There are probably many more co-enzymes for vitamin D yet to be discovered. The co-factor issue is most complex and not too well understood!
Additional co-factors:
“Both vitamin D and A receptors are ubiquitous [ found everywhere at the same time ] not only throughout the eukaryotic kingdom [ organisms whose cells contain complex structures enclosed within membranes ], but also found in EVERY cell of every plant and animal organisms. Scientists continue to locate these receptors in the most interesting places. Thus it may reflect why they are found e-v-e-r-y-w-h-e-r-e. Both vitamins D and A receptors are ubiquitous not only throughout the eukaryotic kingdom, but also found in EVERY cell of every eukaryotic cell. Scientists continue to locate these receptors in the most interesting places [ like human sperm – discovered in 2006 Corbett: NIH info 2006. These receptors appear to be super-switches for energy production and balance, growth, development, reproduction, survival, and life extension.” Minerals & disease
Example of Supplement Vitamin D with co-factors and coenzymes: sold by Biotech Pharmaceutical Inc., is Vitamin D3 Plus TM:

|
ALERT: Sorochan did a local San Diego area survey of pharmacies and health food stores selling vitamin D. The label descriptions indicated that none of the vitamin D supplements had a balance of co-factors. Since the makers of supplements are behind in the research about cofactor balancing, they do not do independent research and hence, the results of their research cannot be trusted. This is an area in need of immediate independent research. |
Warning: The amount of vitamin D in the above label illustration is much higher than the prudent suggestion of "2000 IU of vit D/day." The exact amount of the co-factor mix is probably unknown at this time, so the formula may be a secret guess. You should not take the above supplement without the guidance and supervision of your medical doctor.
Missing information about co-factors:
-- All co-factors for many metabolic processes are unknown at this time.
-- The exact ratio of co-factors to each other is unknown in many instances. For example, how much calcium should be taken at the same time with magnesium?
| Some people retain far too much calcium and are constantly struggling to meet magnesium requirements, while others suffer from magnesium overload and have to supplement larger amounts of calcium to overcome calcium deficiencies. Mineral ratios |
Unless the intracellular status of calcium, magnesium, vitamin K, organic sulfur, zinc or other essential trace elements is measured, it is nearly impossible to predict what exactly will happen to calcium under specific circumstances. Other than the effects of one-sided diets, one-sided supplementation, or organ damage from trauma, infections, or drug use, there are also renal, intestinal and hormonal factors --- all having an impact on someone's mineral status; so there cannot be a fixed mineral ratio that is best for everyone since there are just too many variables. Mineral ratios
For example: the co-factor ratio of [ calcium ] Ca:Mg [ magnesium ] is not a precise ratio. One source suggests a Ca:Mg = 1:1 ratio, based on archeological studies of how man ate and survived over the past 10,000 years. Another source, based on incomplete research, suggests that the ratio may be closer to 2:1 ... that is for example 1000 mg of calcium to 500mg of magnesium. Both ratios take into account that too much magnesium causes diarrhea and, on the other hand, that too much calcium may cause kidney stones and calcification in different parts of the body. These ratios may also be compromised by biochemical individuality. These differences contribute to the controversy over co-factors. Minerals & disease
References:
Cannell John, Vitamin D Newsletter, July 2009, Vitamin D Council. Article no longer active.
Carpenter TO., “ Disturbances of vitamin D metabolism and action during clinical and experimental magnesium deficiency,” Magnesium Research, December 1, 1988, (3-4):131-9. Carpenter: Info Mg 1988
Christensen Stephen, "What Minerals Convert Amino Acids to Dopamine & Serotonin?" LiveStrong.com September 03, 2015. Christensen: Amino Acids to Dopamine & Serotonin 2015
Corbett ST, "Vitamin D receptor found in human sperm,” Urology, December, 2006 (68:6) 1345-9. Corbett: NIH info 2006
Frantom P.A.,et al., “Biochemistry”; Reduction and Oxidation of the Active Site Iron in Tyrosine Hydroxylase: Kinetics and Specificity," February 2006. Frantom: iron & chemistry 2006
Guyenet Stephan. ‘Magnesium and vitamin d metabolism,” Whole Health Source, April 4, 2010. Guyenet: Magnesium and Vitamin D Metabolism 2010
Masterjohn Christopher, "On the Trail of the Elusive X-Factor: A Sixty-Two-Year-Old Mystery Finally Solved," The Weston A. Price Foundation, February 14, 2008. Masterjohn: History of vit K 2008
Medalle R., Waterhouse C., Halur TJ., “ Vitamin D resistance in magnesium deficiency,” American Journal of Clinical Nutrition, August 28, 1976, 29(8):854-8. Mendalle: NIH Mg more info 1976
Mercola Joseph, "A Special Interview with Dr. Cees Vermeer," Mercola: Interview with Dr. Vermeer
Minerals: "Nutrients and Health/Disease," Minerals & disease
Rao, Anuradha, Ralph D. Woodruff, Wendy N. Wade, Timothy E. Kute and Scott D. Cramer, "Genistein and Vitamin D Synergistically Inhibit Human Prostatic Epithelial Cell Growth," J. Nutr. 132:3191-3194, October 2002. Rao: Cofactor genistein and vit D 2002
Reddy V., Sivakumar B.,”Magnesium dependent vitamin D resistant rickets,” Lancet, May 18, 1974, 1(7864):963-5. Reddy: MG and rickets 1974
Rode von Essen Marina, Martin Kongsbak, Peter Schjerling, Klaus Olgaard, Niels Ødum & Carsten Geisler, "Vitamin D controls T cell antigen receptor signaling and activation of human T cells," Nature Immunology 11, 344 - 349 (2010) Published online: 7 March 2010. Rode von Essen: Vit D and receptor cells 2010
Shomon Mary, "Rethinking the TSH Test: An Interview with David Derry, M.D., Ph.D.," Thyroid-Info, March 16, 2011. Shomon: Derry interview
Substrate: In biochemistry, an enzyme substrate is the material upon which an enzyme acts.
Wikipedia, "Calmodulin." Wiki: Calmodulin
Wikipedia, ”Cofactor (biochemistry),” Wiki Cofactor_(biochemistry)
Wikipedia, "1,25-Dihydroxycholecalciferol." Wiki Vit D3 and Ca
Wikipedia, "Holoenzyme Cofactors." Wiki "Holoenzyme Cofactors Wiki Biochemistry/Vitamins_and_Cofactors
Wikipedia, "Nicotinamide adenine dinucleotide." Wiki: NAD
Zofkova Kancheva Rl., “The relationship between magnesium and calciotropic hormones,” Magnesium Research, March 8, 1995 (1):77-84. Zofkova Mg Ca hormones